Scientists Synthesize Extremely Stable Electrocatalyst with Phase-Mixing Strategy
A new way to improve the stability of polymorphic cobalt diselenide (CoSe2) catalyst through phase mixing is reported by the research team led by academician YU Shuhong and professor GAO Minrui. This work is published in Nature Communications on Nov 25th.
Clean energy can break the dependence on fossil fuels and tackle environmental challenges such as climate change. Clean-energy technologies such as fuel cells and electrolyzers require active and stable catalysts that accelerate the electrode reactions.
However, many catalysts degrades in harsh electrochemical environments. This is a major challenge that limits the device effificiency and cost effectiveness. For example, polymer electrolyte membranebased electrochemical devices need low pH environment for operation but the long-term stability of emerging nonprecious metal-based catalysts in acidic media is unsatisfactory.
The research team develop an alkali-heating approach to produce phase-mixed CoSe2 with nearly homogeneous distribution of cubic and orthorhombic phases.
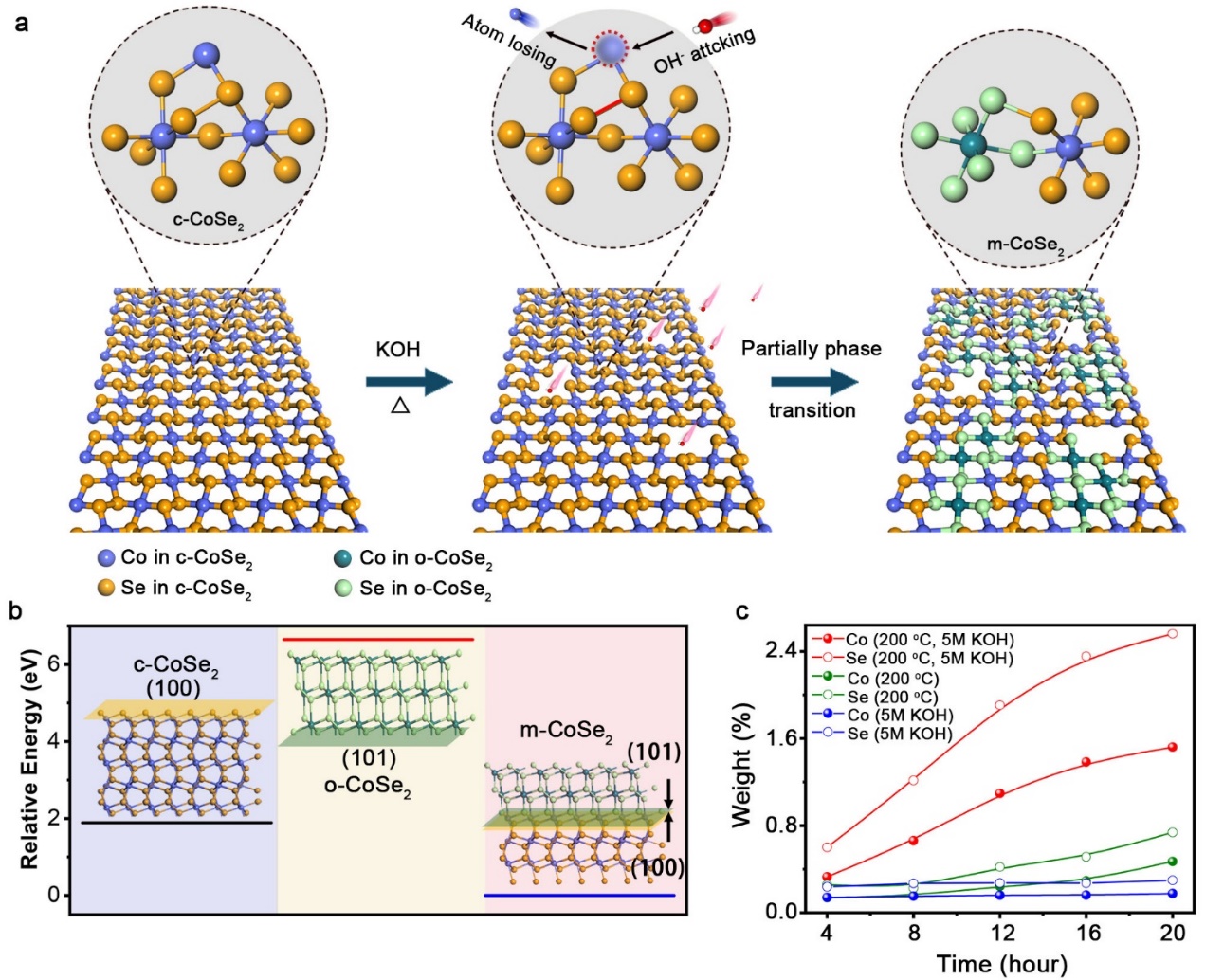
Structural phase transition in cubic CoSe2
While the stabilities of both cubic CoSe2 and orthorhombic CoSe2 are ordinary, phase-mixed CoSe2 is extremely stable for catalyzing hydrogen evolution reaction (HER) in acid. In acidic electrolyte, the novel phase-mixed catalyst shows no sign of deactivation after more than 400 hours of continuous operation and the polarization curve is well retained after 50000 electrochemical cycles.
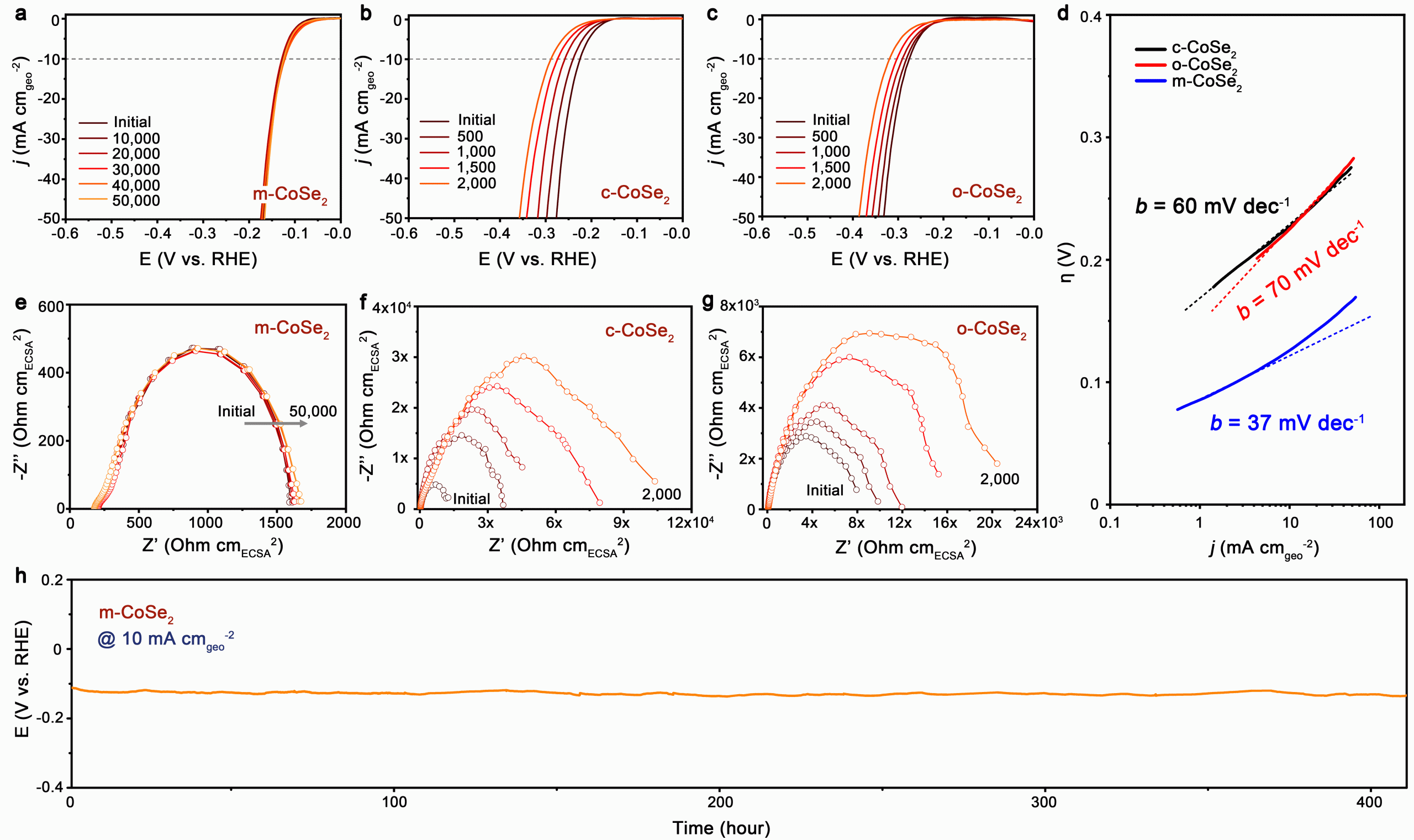
Electrochemical stability of phase-mixed, cubic, and orthorhombic CoSe2
The tremendously enhanced stability of phase-mixed CoSe2 can be explained by its robust lattice and the greater covalency of Co-Se bonds after phase mixing.
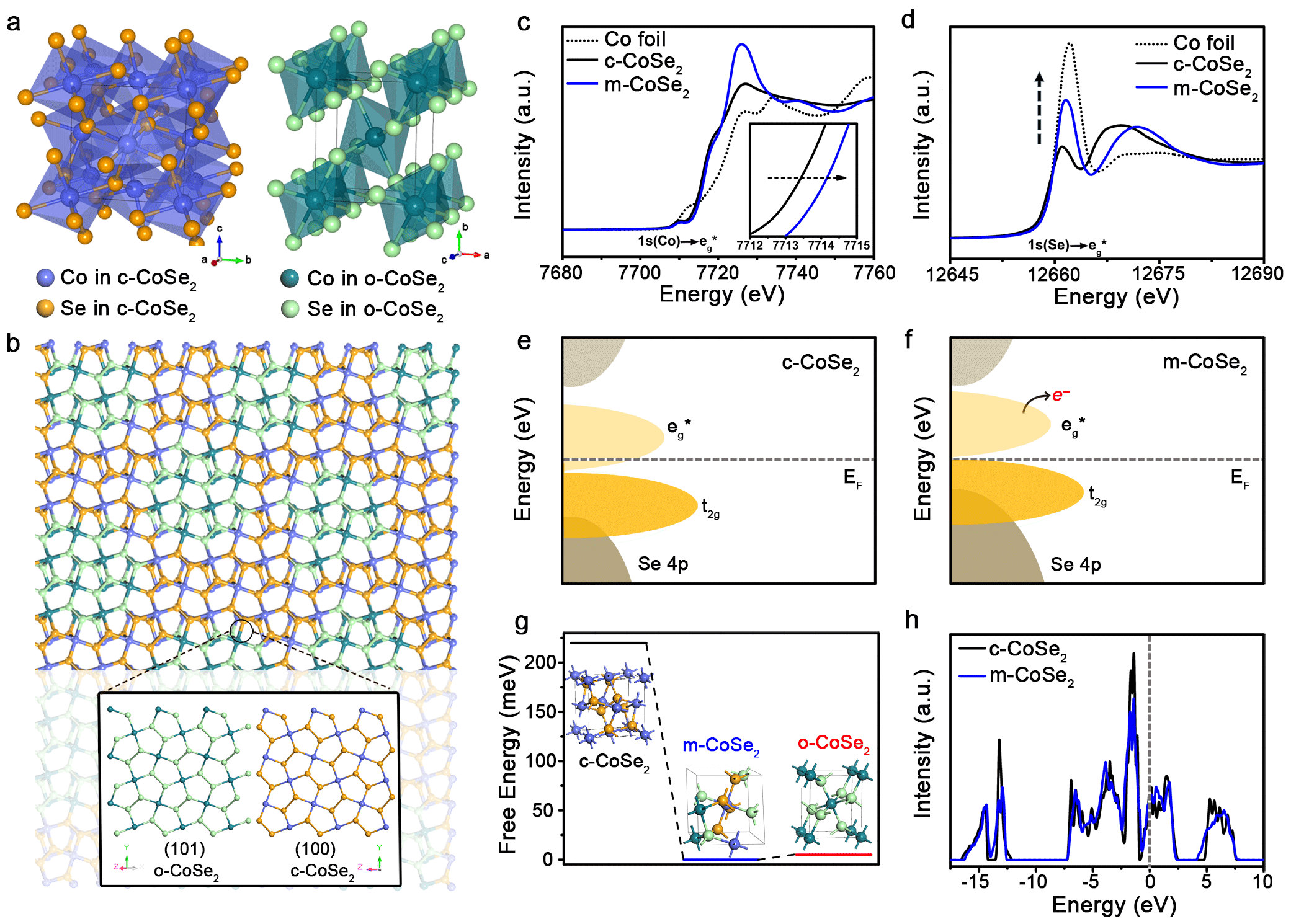
Density functional theory (DFT) calculation and stability mechanism
The findings provide promising design strategy for long-lived catalysts in acid through crystal phase engineering. The phase mixing method could be applicable to other polymorphic materials for designing new electrocatalysts with better performances.
Paper link:
https://www.nature.com/articles/s41467-019-12992-y
(Written by YANG Ziyi, edited by LU Hongyu, USTC News Center)

