Researchers Reveal The Atomic Structure Of The Human Herpesvirus 6B Capsid
Prof.BI Guoqiang's team obtained the near-atomic resolution structure of the human herpesvirus 6B capsid and capsid-associated tegument complexes. The study was published in Nature Communications.
Human herpesvirus 6B (HHV-6B) belongs to the β-herpesvirus subfamily of the Herpesviridae. Difficulties in cultivating HHV-6B have prevented high-resolution structural studies and limited our understanding of its assembly.
HHV-6 infects nearly all human beings by the age of three and often results in fever, diarrhea, and the roseola rash. Herpesviruses—such as herpes simplex, chicken pox, and the Epstein–Barr virus—have a tendency of establishing life-long latency, activating later in life with many clinical manifestations; HHV-6 follows a similar cycle. HHV-6 reactivation in brain tissue can cause cognitive dysfunction, permanent disability, and death.
In the study, researchers have employed a plethora of cutting-edge cryoEM techniques and a sub-particle reconstruction method to work with very little and minimally purified HHV-6B sample and obtained the near-atomic resolution structure of HHV-6B. The results showed a capsid-binding pattern of pU11 that differs from capsid-associated tegument complex (CATC) binding patterns found in human cytomegalovirus (HCMV) and murine cytomegalovirus (MCMV).
They determined the near-atomic resolution structure of HHV-6B using crude, minimally purified samples, derived the atomic model of both the HHV-6B capsid and its capsid-associated tegument complex of pU11 tetramer. This represents the fifth-ever human herpesvirus whose capsid structure has been described at atomic level, following HCMV, herpes simplex virus 1 and 2. HHV-6B’s capsid structure is similar to known capsid structures of other herpesviruses with only minor differences.
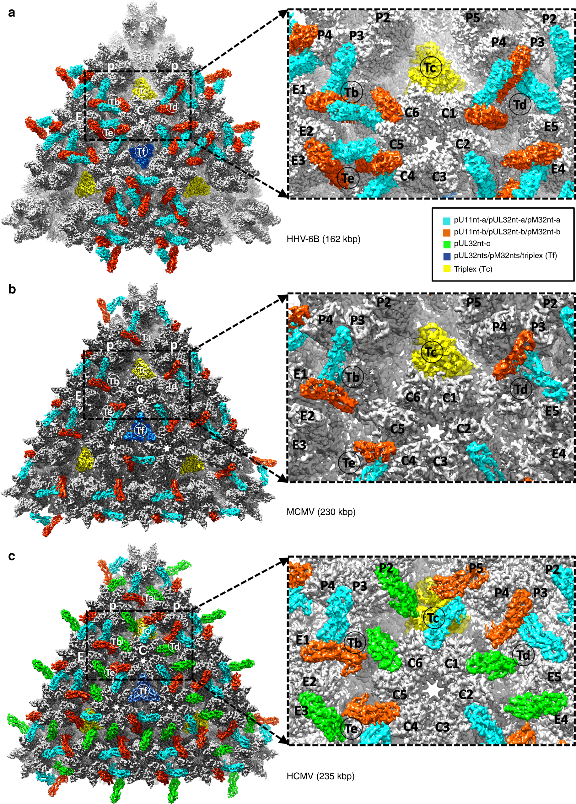
Comparison of CATC-capsid-binding patterns in HHV-6B, MCMV, and HCMV. (Image by Yibo Zhang et al.)
The atomic model revealed that binding between capsid-vertex specific components (CVSC) and the capsid of herpesviruses is not determined solely by the availability of triplex binding sites on the capsid. It is natural to expect the binding pattern of CVSC tegument proteins on pentons to be dictated by the availability of its binding partners.
They predicted that CVSC complexes are present in HHV-6B, but only bind to the portal vertex and peri-portal triplexes. Owing to the importance of CVSC in viral propagation, fully understanding the nature of CVSC binding could prove valuable towards the development of pharmaceutical drugs.
Looking forward, the significance of the atomic structure of HHV-6B transcends basic knowledge about HHV-6B capsid and tegument assembly.
Protein phosphorylation has been recognized as a common mechanism for regulating biological functions. Drugs against infection, inflammation, cancers, or neurodegeneration target phosphorylation pathways of human proteins; for example, searching for kinase inhibitors has been one of the major focuses of drug development during the past two decades. Understanding the atomic structure of the phosphorylated protein pU11 in HHV-6B may prove vital to designing anti-viral drugs and vaccines against this virus.
The reseach is in cooperation with the team led by professor ZHOU Zhenghong from University of California and MEI Ye from East China Normal University,
(Written by LI Xiaoxi, edited by LU Hongyu, USTC News Center)

