USTC Obtained New Progress in the Research on Graphene Ion Storage Mechanism
-
Electrochemical double layer capacitors (EDLCs), also known as super capacitors, store energy by reversible electrostatic attraction of electrolyte ions onto high surface area carbon electrodes. Since the limitation of battery-like charge transfer kinetics is not involved, super capacitors can operate at very high charge and discharge rates within a few seconds, and can have excellent cyclability of over a million cycles, which make them useful in a broad range of energy applications.Graphene with its high theoretical specific surface area of 2630 m2/g and theoretical capacity of up to 550 F/g, has already attracted great attention for super capacitor applications.Despite efforts mentioned above, the current performances achieved by graphene-based materials are still far from expectations.The dynamic charge separation mechanism of the graphene/electrolyte interface has not been well solved, which hinders the further development of high-performance two-dimensional or three-dimensional graphene electrodes.
Recently, the research team led by Prof. ZHU Yanwu from the University of Science and Technology of China (USTC), has found that a positively charged ion-species desorption and an ion re-organization dominate the double layer charging during positive and negative polarizations, respectively, leading to the increase in electrical double-layer (EDL) capacitance with applied potential. The research named Charge Storage Mechanisms of Single Layer Graphene in Ionic Liquid was published in Journal of the American Chemical Society on October 5th.
In the research, electrochemical impedance spectroscopy (EIS) and electrochemical quartz crystal microbalance (EQCM) are used to characterize the ion fluxes and adsorption on single layer graphene in neat ionic liquid (EMI-TFSI) electrolyte.The researchers delivered a qualitative demonstration of the combination of EQCM and single layer graphene (SLG) in neat EMI-TFSI. Scanning from potential of zero charge (PZC) towards positive charge, positively charged ion-species were expelled. Yet, from PZC to negative charge, the orientation of ions changed, resulting in more compact packing.
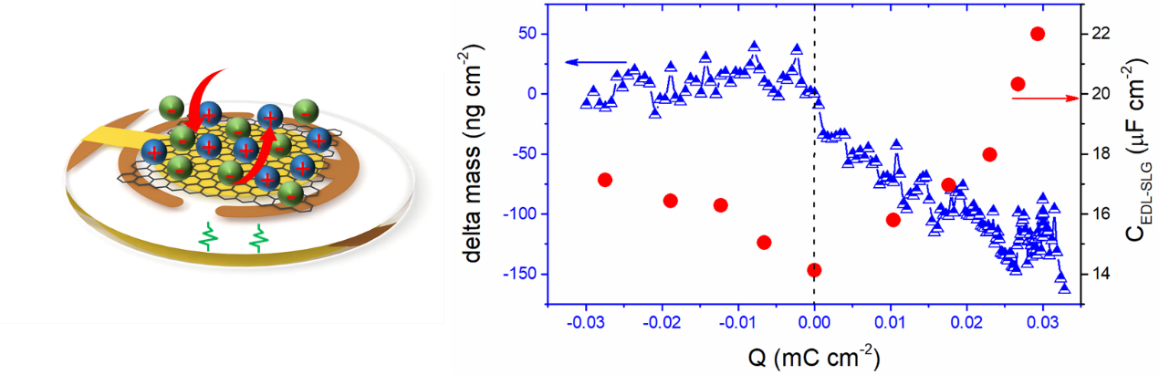
Fig1: Quartz crystallite balance for in situ electrochemical detection schematic (left), observed graphene surface ion response (right).
These results also suggest a surface-bound ions dominated charging process on SLG in neat IL, which is completely different from that of porous carbons.18,37,41 It also highlights the important role of electrostatic correlations on ion fluxes and ion separation at a non-porous carbon interface, introduced by long-range Coulombic force.
During the work, the research team cooperated with the French Patrice Simon group. The kinetic response of ionic liquid (EMI-TFSI) electrolyte on the surface of single-layer graphene was studied in situ by electrochemical impedance spectroscopy combined with electrochemical quartz crystal microbalance system. It is found that in the graphene anodization interval, the charge storage is dominated by the desorption of positively charged cluster ions; in the negative polarization region, the surface quality of graphene changes little, showing the surface ion rearrangement effect.As the applied potential increases, the two types of interface responses dominate the changes in the electric double layer, resulting in an increase in the electric double layer capacitance.This study provides a basis for further understanding of the graphene-electrolyte interface structure and graphene double layer energy storage.
In the future, further investigations are needed to enable a better understanding of the detailed capacitance response at graphene/ionic liquid interface.Also, researchers expect with the extension of this work, from single layer to multi-layered graphene, the fundamental ion dynamics of graphene-based materials can be deeply investigated in the future.
The research was supported by The National Natural Science Foundation of China, the Agence Nationale de la Recherche, and the China Scholarship Council (CSC) program.
Paper link:
https://pubs.acs.org/doi/10.1021/jacs.9b07134
(Written by LI Xiaoxi, edited by LU Hongyu, USTC news center)

